Abstract Lipids have physical, chemical, and physiological properties that make them important factors in human nutrition. They form a group of compounds of varied chemical nature that have the common property of being soluble in organic solvents but insoluble in water. This basic property affects their digestion, absorption, and transport in the blood and metabolism at cellular level. Firstly, fatty-acid chain length and number of double bonds influence fat absorption. Thus, medium-chain fatty acids (MCFA) are better absorbed than long-chain fatty acids. Secondly, the positional distribution of fatty acids (FA) in dietary triglycerides (TG) determine whether FA are absorbed as 2-monoglycerides (2- MG) or free fatty acids (FFA), and hence, influences the composition of chylomicroms (CM) because triglycerides (TG) are resyntethysed in the intestinal mucosa using 2-MG from dietary lipids. Generally, the absorption of FA in the sn-2 position of TG is favored, whereas no specificity has been found for the fatty acids in the sn-1 and sn-3 positions. Finally, some FA of nutritional interest, namely, long-chain polyunsaturated fatty acids (LCP), are present in dietary lipid sources as both TG or phospholipids (PL). Fatty acids esterified as PL or TG may show different availability. In fact, some authors have suggested a better absorption of LCP-PL. Moreover, dietary LCP in form of TG or PL differently affects the composition of HDL and LDL PL. D 2001 Elsevier Science Ireland Ltd. All rights reserved. Keywords: Long-chain poly
Category: News
Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals.
Abstract
Short chain fatty acids (SCFA) also named volatile fatty acids, mainly acetate, propionate and butyrate, are the major end-products of the microbial digestion of carbohydrates in the alimentary canal. The highest concentrations are observed in the forestomach of the ruminants and in the large intestine (caecum and colon) of all the mammals. Butyrate and caproate released by action of gastric lipase on bovine milk triacylglycerols ingested by preruminants or infants are of nutritional importance too. Both squamous stratified mucosa of rumen and columnar simple epithelium of intestine absorb readily SCFA. The mechanisms of SCFA absorption are incompletely known. Passive diffusion of the unionized form across the cell membrane is currently admitted. In the lumen, the necessary protonation of SCFA anions could come first from the hydration of CO2. The ubiquitous cell membrane process of Na+-H+ exchange can also supply luminal protons. Evidence for an acid microclimate (pH = 5.8-6.8) suitable for SCFA-protonation on the surface of the intestinal lining has been provided recently. This microclimate would be generated by an epithelial secretion of H+ ions and would be protected by the mucus coating from the variable pH of luminal contents. Part of the absorbed SCFA does not reach plasma because it is metabolized in the gastrointestinal wall. Acetate incorporation in mucosal higher lipids is well-known. However, the preponderant metabolic pathway for all the SCFA is catabolism to CO2 except in the rumen wall where about 80% of butyrate is converted to ketone bodies which afterwards flow into bloodstream. Thus, SCFA are an important energy source for the gut mucosa itself.
Absorption in the Stomach
Absorption[edit]
Although the absorption in the human stomach is mainly a function of the small intestine, some absorption of certain small molecules nevertheless does occur in the stomach through its lining. This includes:
- Water, if the body is dehydrated
- Medication, like aspirin
- Amino acids[10]
- 10–20% of ingested ethanol (e.g. from alcoholic beverages)[11]
- Caffeine[12]
- To a small extent water-soluble vitamins (most are absorbed in the small intestine)[13]
The parietal cells of the human stomach are responsible for producing intrinsic factor, which is necessary for the absorption of vitamin B12. B12 is used in cellular metabolism and is necessary for the production of red blood cells, and the functioning of the nervous system.
How Are Nutrients Absorbed in the Body by the Stomach?
Food provides the fuel you need to perform all functions of living. The nutrients that food gives you, from vitamins to proteins, have specific functions in the body that keep you healthy and support your body’s systems. But your body cannot use the nutrients you eat until it breaks them down in small pieces to then absorb them. Your stomach plays a crucial role in digestion and absorption, but it is only a part of the larger digestive system.
The Digestive System

The stomach is the third stop for food along the digestive tract. The digestive tract begins in your mouth and goes down through the esophagus, continuing into the stomach, small intestine, large intestine, rectum and then ending at the anus, where waste is eliminated. The digestive tract also includes a layer of smooth muscle that moves the food from the beginning to the end of the tract. Without the muscle, the food would not be able to get from the esophagus to the stomach. The entire digestive tract is approximately 30 feet long in an adult. The pancreas and liver also assist digestion by making digestive juices the body uses to break down food. The gallbladder stores the digestive juices the liver makes.
Digestion

Digestion is a necessary part of the absorption process. Food first enters the stomach after you chew and swallow it. The stomach stores the food and liquid you swallow and mixes it with digestive juices that the stomach makes. The muscles of the stomach mix the contents up to break them down into smaller pieces so the nutrients can be absorbed. Nutrients include vitamins, minerals, fats, carbohydrates and proteins. Different types of nutrients need more or less time to be processed by the stomach and small intestine before being absorbed through the intestinal walls. Carbohydrates require the least amount of time in the stomach to digest. Protein needs more time and fats take the longest.
Nutrient Absorption

The stomach breaks food down and passes it to the small intestine. Nutrients enter the bloodstream through fingerlike projections called villi that are along the inner wall of the small intestine. Your body absorbs most of the nutrients during the process of moving the food from the stomach to the small intestine, but the large intestine does absorb some nutrients. The main job of the large intestine is actually to remove water from undigested matter and to form solid waste for your body to excrete. However, the colon, which is part of the large intestine, does contain bacteria that assist in digesting any remaining food that reaches the large intestine.
Elimination

The rectum is at the end of the digestive tract. It is part of the large intestine. The rectum stores feces until you have a bowel movement and release waste products through the anus. Your body removes all the nutrients it can from food and absorbs them before the food reaches the rectum. Anything left over your body can’t use and therefore eliminates.
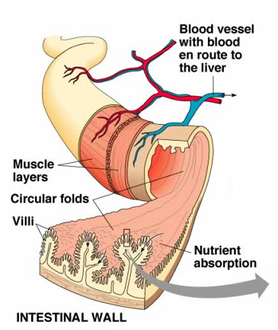
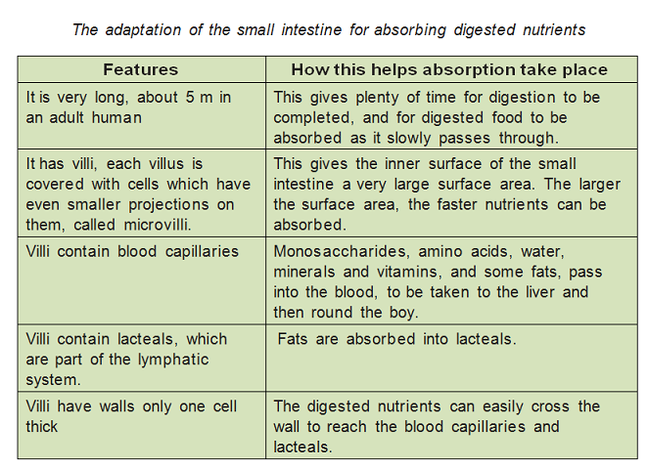
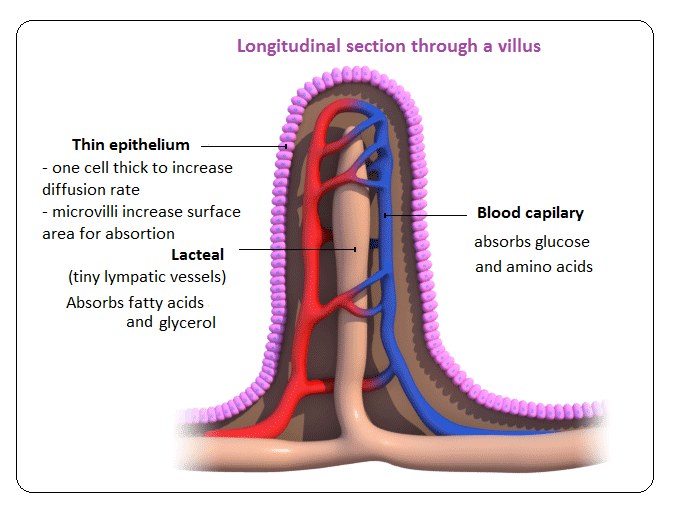
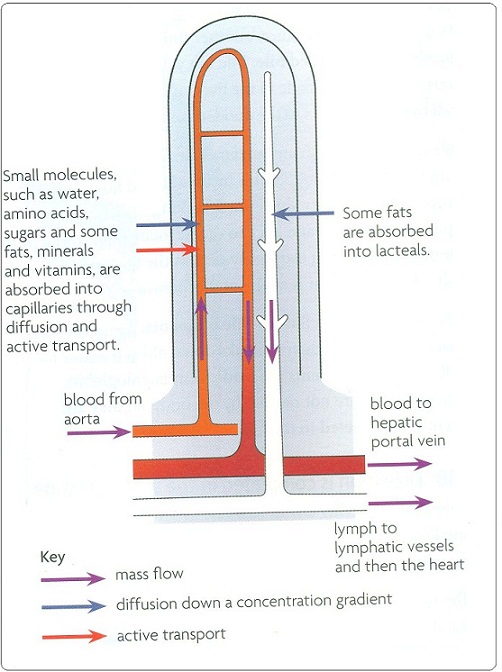
Absorbtion – function of the small intestine and significance of villi
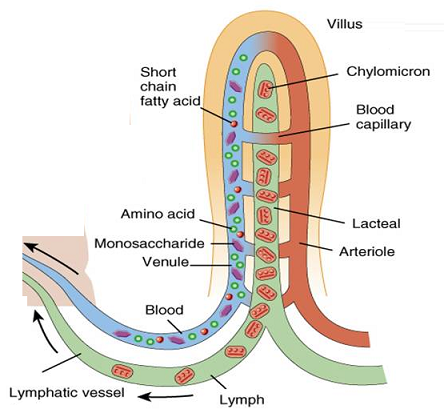
Villi are finger like projections that increase the surface area for absorption. If a section of small intestine was turned inside out, its surface would be kike a carpet. Inside each villus are:
– Blood capillaries: absorb amino acids and glucose.
– Lacteals: absorb fatty acids and glycerol.
– mainly by diffusion.
– or by active transport.
Epithelial cells contain mitochondria to provide energy for absorption against the concentration gradient.
The hepatic portal vein transports absorbed food from the small intestine to the liver. After a meal, the blood in this vein contains very high concentrations of glucose and amino acids, as well as vitamins and minerals. The liver reduces levels backs to normal.
Ruminant Anatomy and Physiology
Anatomy of the Adult

The cow’s digestive tract consists of the mouth, esophagus, a complex four-compartment stomach, small intestine and large intestine (figure 1). The stomach includes the rumen or paunch, reticulum or “honeycomb,” the omasum or “manyplies,” and the abomasum or “true stomach.”
The rumen. The rumen (on the left side of the animal) is the largest of four compartments and is divided into several sacs. It can hold 25 gallons or more of material, depending on the size of the cow. Because of its size, the rumen acts as a storage or holding vat for feed. It is also a fermentation vat. A microbial population in the rumen digests or ferments feed eaten by the animal. Conditions within the rumen favor the growth of microbes. The rumen absorbs most of the volatile fatty acids produced from fermentation of feedstuffs by rumen microbes. Absorption of volatile fatty acids and some other products of digestion is enhanced by a good blood supply to the walls of the rumen. Tiny projections called papillae increase the surface area and the absorption capacity of the rumen.
The reticulum. The reticulum is a pouch-like structure in the forward area of the body cavity. The tissues are arranged in a network resembling a honeycomb. A small fold of tissue lies between the reticulum and the rumen, but the two are not actually separate compartments. Collectively they are called the rumino-reticulum. Heavy or dense feed and metal objects eaten by the cow drop into this compartment. The reticulum lies close to the heart. Nails and other sharp objects may work into the tissue and cause “hardware disease.” If not prevented by a magnet or corrected by surgery, infection may occur and the animal may die.
The omasum. This globe-shaped structure (also called the “manyplies”) contains leaves of tissue (like pages in a book). The omasum absorbs water and other substances from digestive contents. Feed material (ingesta) between the leaves will be drier than that found in the other compartments.
The abomasum. This is the only compartment (also called the true stomach) with a glandular lining. Hydrochloric acid and digestive enzymes, needed for the breakdown of feeds, are secreted into the abomasum. The abomasum is comparable to the stomach of the non-ruminant.
The small intestine. The small intestine measures about 20 times the length of the animal. It is composed of three sections: the duodenum, jejunum, and ileum. The small intestine receives the secretions of the pancreas and the gallbladder, which aid digestion. Most of the digestive process is completed here, and many nutrients are absorbed through the villi (small finger-like projections) into the blood and lymphatic systems.
Cecum. The cecum is the large area located at the junction of the small and large intestine, where some previously undigested fiber may be broken down. The exact significance of the cecum has not been established.
Large intestine. This is the last segment of the tract through which undigested feedstuffs pass. Some bacterial digestion of undigested feed occurs, but absorption of water is the primary digestive activity occurring in the large intestine.
Function of the Digestive Tract
Eructation (belching). Large quantities of gas, mostly carbon dioxide and methane, are produced in the rumen. Production amounts to 30 to 50 quarts per hour and must be removed; otherwise bloating occurs. Under normal conditions, distension from gas formation causes the cow to belch and eliminate the gas.
Rumination. A cow may spend as much as 35 to 40 percent of each day ruminating (cud chewing). The actual amount of time spent ruminating varies from very little (when grain or finely ground rations are fed) to several hours (when long hay is fed). Mature cattle spend little time chewing when eating. During rest periods, feed boluses (cud) are regurgitated for rechewing to reduce particle size and for resalivation. Feed is more readily digested by rumen microbes as particle size is reduced.
Motility of the rumen and reticulum. The rumen is always contracting and moving. Healthy cows will have one to two rumen contractions per minute. The contractions mix the rumen contents, bring microbes in contact with new feedstuffs, reduce flotation of solids, and move materials out of the rumen. Lack of or a decrease in frequency of rumen movements is one way of diagnosing sick animals.
Saliva production. As much as 50 to 80 quarts of saliva can be produced by salivary glands and added to the rumen each day. Saliva provides liquid for the microbial population, recirculates nitrogen and minerals, and buffers the rumen. Saliva is the major buffer for helping to maintain a rumen pH between 6.2 and 6.8 for optimum digestion of forages and feedstuffs.
Vomiting. Cattle rarely vomit. Occasionally certain feeds will induce vomiting. Some pasture plants, usually weeds, contain alkaloids that can cause this problem. Should this condition persist, a veterinarian should be consulted.
 Figure 2. Microbial digestion of feed carbohydrate
Figure 2. Microbial digestion of feed carbohydratein the rumen.
Digestion of energy feeds in the rumen. Simple and complex carbohydrates (fiber) are digested by rumen microbes and converted into volatile fatty acids. The volatile fatty acids, which consist mainly of acetic, propionic, and butyric acids, are the primary energy source for ruminants (figure 2). When large amounts of forage are fed, the formation of acetic acid predominates (60 to 70 percent of total) with lesser amounts of propionic (15 to 20 percent) and butyric (5 to 15 percent) acids occurring. However, when grain feeding is increased or when finely ground forages are fed, the proportion of acetic acid may decrease to 40 percent, while the amount of propionic acid may increase to 40 percent. Such a change in volatile fatty acid production generally is associated with a reduction in milk fat test.
Approximately 30 to 50 percent of the cellulose and hemicellulose is digested in the rumen by the microbial population. Sixty percent or more of the starch is degraded, depending on the amount fed and how fast ingested materials move through the rumen. Most sugars are 100 percent digested within the rumen.
The volatile fatty acids are absorbed from the rumen into the blood stream and transported to body tissues, including the udder, where they are used as sources of energy for maintenance, growth, reproduction, and milk production. The cow derives 50 to 70 percent of its energy from the volatile fatty acids produced in the rumen.
 Figure 3. Schematic summary of nitrogen utilization by the
Figure 3. Schematic summary of nitrogen utilization by theruminant. Source: Satter, 1978. Minnesota Nutrition
Conference Proceedings.
Protein and nonprotein nitrogen utilization in the rumen. Some of the protein consumed by the cow escapes breakdown in the rumen (figure 3). Protein undergoing fermentation is converted to ammonia, organic acids, amino acids, and other products. Approximately 40 to 75 percent of the natural protein in feed is broken down. The extent of breakdown depends on many factors including solubility of the protein, resistance to breakdown, rate of feed passage through the rumen, and others. Many rumen micro-organisms require ammonia (breakdown product of protein) for growth and synthesis of microbial protein. Ammonia also may be provided from NPN sources such as urea, ammonium salts, nitrates, and other compounds. Rumen microbes convert the ammonia and organic acids into amino acids that are assembled into microbial protein. Excess ammonia is mostly absorbed from the rumen into the blood stream, but small amounts may pass into the lower digestive tract and be absorbed. Feed protein (that escapes breakdown in the rumen) and microbial protein pass to the abomasum and small intestine for digestion and absorption.
Vitamin synthesis. The rumen micro-organisms manufacture all of the B vitamins and vitamin K. Vitamin synthesis in the rumen is sufficient for growth and maintenance. Under most conditions, cattle with functioning rumens do not require supplemental B vitamins or vitamin K in the diet. Niacin (B3) and thiamine (B1) may be needed under stress conditions.
Fat digestion. Most of the digestion and absorption of fat occurs in the small intestine. Rumen micro-organisms change unsaturated fatty acids to saturated acids through the addition of hydrogen molecules. Thus, more saturated fat is absorbed by cows than by simple-stomach animals. Feeding large quantities of unsaturated fatty acids can be toxic to rumen bacteria, depress fiber digestion, and lower rumen pH.
Calf Digestive System
At birth and during the first few weeks of life, the rumen, reticulum, and omasum are undeveloped. In contrast to the mature cow, in the calf, the abomasum is the largest compartment of the stomach (table 1). At this stage of life, the rumen is nonfunctional and some feeds digested by the adult cannot be used by the calf. During nursing or feeding from a bucket, milk bypasses the rumen via the esophageal groove and passes directly into the abomasum. Reflex action closes the groove to form a tube-like structure which prevents milk or milk replacer from entering the rumen. When milk is consumed very rapidly, some may overflow into the rumen.
As long as the calf remains on milk, the rumen remains undeveloped. When calves begin consuming grain and forage, a microbial population becomes established in the rumen and reticulum. End products of microbial fermentation are responsible for the development of the rumen. This occurs as early as 3 weeks of age with most feeding programs. Cud inoculation is not necessary to initiate rumen development. If grain feeding with or without forage is started during the first few weeks of life, the rumen will become larger and heavier with papillae development, and will begin functioning like the adult’s when the calf is about 3 months of age.
Alternatives to Colistin Sulphate & ZnO
Do you believe the following can replace Colistin Sulphate & ZnO? Do you believe you can see the solid results within 3-7 days?
- Intestinal astringents – Tanpro and Colistizer ;
- Green & Broad-spectrum Intestinal Antimicrobial to replace Coistin Sulphate: Superstin (Benzoic Acid) ;
- Anti-secretory antidiarrheal agent: Buty-ER (Calcium Butyrate).
Insighter® is committed to customers’ success in Livestock GUT Health by providing non-antibiotic antidiarrheal solutions whose results can be seen within 3-7 days. With our second-generation NGPs (Alternatives to AGPs), we are able to help feed mills and farms get rid of their headaches of diarrheal incidence with the above-mentioned legitimate, efficacious and relatively cost-effective products.
Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level
Alip Borthakur, Ravinder K. Gill, Kim Hodges, Krishnamurthy Ramaswamy, Gail Hecht, and Pradeep K. Dudeja from Section of Digestive Diseases and Nutrition, Department of Medicine, University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center, Chicago, Illinois
Enteropathogenic Escherichia coli (EPEC), a food-borne human pathogen, is responsible for infantile diarrhea, especially in developing countries. The pathophysiology of EPEC-induced diarrhea, however, is not completely understood. Our recent studies showed modulation of Na + /H + and Cl – /HCO 3 – exchange activities in Caco-2 cells in response to EPEC infection. We hypothesized that intestinal short-chain fatty acid absorption mediated by monocarboxylate transporter 1 (MCT1) might also be altered by EPEC infection. The aim of the current studies was to examine the effect of EPEC infection on butyrate uptake. Caco-2 cells were infected with wild-type EPEC, various mutant strains, or nonpathogenic E. coli HS4, and butyrate uptake was determined. EPEC, but not nonpathogenic E. coli, significantly decreased butyrate uptake. Infection of cells with strains harboring mutations in escN, which encodes a putative ATPase for the EPEC type III secretion system (TTSS), or in the espA, espB, or espD genes encoding structural components of the TTSS, had no effect on butyrate uptake, indicating the TTSS dependence. On the other hand, strains with mutations in the effector protein genes espF, espG, espH, and map inhibited butyrate uptake, similar to the wild-type EPEC. Surface expression of MCT1 decreased considerably after EPEC but not after nonpathogenic E. coli infection. In conclusion, our studies demonstrate inhibition of MCT1-mediated butyrate uptake in Caco-2 cells in response to EPEC infection. This inhibition was dependent on a functional TTSS and the structural proteins EspA, -B, and -D of the translocation apparatus.
【Keywords】 short-chain fatty acids ion transport diarrhea monocarboxylate transporter
BUTYRATE AND OTHER SHORT – CHAIN fatty acids (SCFAs) are produced by enteric bacterial fermentation of undigested carbohydrates and dietary fiber. SCFAs are avidly absorbed in the colon and serve as preferential fuel for colonic epithelial cells. Butyrate is the most important SCFA in colonocyte metabolism and is used preferentially over propionate and acetate. We have previously shown ( 12 ) that SCFA absorption across the epithelial membranes of the human ileum and colon involves an SCFA/HCO 3 – (OH – ) exchange mechanism. We and others also demonstrated the involvement of monocarboxylate transporter 1 (MCT1) in the luminal absorption of SCFAs in human intestinal epithelial cells ( 11, 28 ). Butyrate is known to stimulate water and NaCl absorption via activation of Na + /H + exchanger (NHE; see Refs. 6 and 25 ) and apical Cl – /HCO 3 – exchangers ( 24 ). The anti-inflammatory actions of butyrate are supported by both clinical and animal studies, implicating its role in suppressing mucosal inflammation ( 16 ). Also, butyrate-containing retention enemas have proven beneficial in the treatment of ulcerative and diversion colitis ( 6 ). Although decreased SCFA production resulting from impaired colonic fermentation has been shown to impair colonic absorption of sodium and water, little information is available on the factors that might influence efficient absorption of SCFAs by colonocytes. Particularly, no information is available on the modulation of SCFA uptake by pathogenic microorganisms that cause secretory and inflammatory diarrhea in humans.
Enteropathogenic Escherichia coli (EPEC) is an important noninvasive human enteric pathogen associated with diarrhea, particularly in infants. Its infection causes specific histopathological alterations of the intestinal enterocytes, called attaching and effacing (A/E) lesions, characterized by effacement of microvilli, close adherence of the bacteria to the host cell membrane, and recruitment of filamentous actin and other cytoskeletal proteins resulting in pedestal formation beneath the sites of attachment ( 32 ). The gene products required for producing A/E lesions are encoded by an 35-kb pathogenicity island in the bacterial chromosome, known as the Locus of Enterocyte Effacement (LEE). The LEE encodes a number of virulence genes, including the components of a type III secretion system (TTSS) that allows direct transfer of bacterial effector molecules into host cells ( 22 ). Several effectors, which are translocated into host cells via the TTSS, have been identified and characterized ( 21 ). These include Tir, EspF, EspG, EspH, and Map. The complete mechanism(s) of EPEC-induced diarrhea is not fully understood and appears to be multifactorial. One such factor may be impairment of ion and solute transport. Diarrhea associated with infection by enteric pathogens could result from either increased Cl – secretion, decreased NaCl absorption, or both. We have previously shown that Cl – /OH – exchange activity is inhibited in response to EPEC infection in Caco-2 cells ( 13 ). Also, EPEC infection increased the activity of NHE2, whereas the activity of NHE3, the predominant Na + -absorbing isoform, was inhibited ( 14 ). Based on the fact that EPEC-induced diarrhea is multifactorial, and that SCFAs maintain mucosal integrity and influence water and electrolyte absorption, it was of interest to determine if butyrate absorption by mucosal cells is affected by EPEC infection. We report here that butyrate uptake by intestinal epithelial Caco-2 cells is significantly decreased by EPEC infection. We further show that membrane expression of MCT1, the SCFA transporter in the intestinal epithelial cells, is also decreased in response to EPEC infection.
MATERIALS AND METHODS
Materials. sodium butyrate was obtained from NEN Life Science Products (Boston, MA). Caco-2 cells were obtained from ATCC (American Type Culture Collection, Manassas, VA). Sulfo-NHS-SS-biotin for biotinylation of cell surface proteins and streptavidin agarose were from Pierce (Rockford, IL). All other reagents were of at least reagent grade and were obtained from Sigma (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
Cell culture. Caco-2 cells were grown at 37°C in an atmosphere of 5% CO 2. Cells were maintained in DMEM with 4.5 g/l glucose, 50 U/ml penicillin, 5 µg/ml streptomycin, 2 µg/ml gentamicin, and 20% FBS. Cells used for these studies were plated on 24-well plates at a density of 2 x 10 4 cells/well. Cells were used for bacterial infection and butyrate uptake on day 12-14 postplating and were grown overnight in a medium free of serum and antibiotic before infection.
Bacterial culture and infection of cells. The EPEC strains used in this study were as follows: wild-type EPEC strain E2348/69, CVD452 (E2348/69 escN :: Kanamycin; see Ref. 17 ), UMD864 (E2348/69 D48-759 espB1; see Ref. 7 ), UMD 870 (E2348/69 espD1 :: aph-3; Km; see Ref. 20 ), and E2348/69 espG orf3 ( 8 ) and the nonpathogenic isolate HS4 (obtained from J. B. Kaper’s laboratory). Strains were grown overnight in Milleva Luria Borth (LB) media. On the day of experiment, an aliquot of the overnight culture was inoculated in an appropriate volume of serum and antibiotic-free medium supplemented with 0.5% mannose. Bacteria were grown to midlog phase (optical density at 600 nm = 0.4). The culture was spun down and resuspended in the same volume of fresh media. Cell monolayers were then infected at a multiplicity of infection of 100. After infection for the desired time, media were removed, and cell monolayers were washed with PBS.
butyrate uptake was determined using cold butyrate concentrations ranging from 0.5 to 15.0 mM.
Biotinylation of cell surface proteins and Western blot. Biotinylation of cell surface proteins was carried out according to Akhter et al. ( 1 ) with minor modifications. Briefly, EPEC-infected or control cell monolayers were washed three times with PBS containing 0.1 mM CaCl 2 and 1 mM MgCl 2 at 4°C. The apical surface was then exposed to Sulfo-NHS-SS-biotin (Pierce) at a concentration of 1.5 mg/ml in borate buffer, pH 9.0, by incubation for 1 h at 4°C in horizontal motion. Cells were then quenched with PBS containing CaCl 2, MgCl 2, and 100 mM glycine for 20 min at 4°C. Cells were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris·Cl, pH 7.4, 5 mM EDTA, 1% Triton X-100, 0.1% SDS, and protease inhibitor cocktail). After centrifugation, the supernatant was incubated overnight in streptavidin agarose and then washed three times with lysis buffer. The streptavidin agarose beads were spun down, and sample loading buffer for SDS-PAGE was added. Separated proteins were probed with anti-MCT1 antibody and visualized with enhanced chemiluminescence reagent.
Statistical analysis. Results are expressed as means ± SE. Each independent set of results represents data from at least six wells used in three to five separate experiments.
RESULTS
EPEC infection inhibits butyrate uptake in Caco-2 cells. To determine whether EPEC infection had any effect on butyrate uptake, Caco-2 cells were infected for 1 h, and butyrate uptake was determined as pH-driven butyrate uptake, as described in MATERIALS AND METHODS. Figure 1 shows that butyrate uptake was decreased significantly ( 60%) in EPEC-infected cells compared with uninfected controls. In contrast, infection with nonpathogenic E. coli had no significant effect.
Fig. 1. Enteropathogenic Escherichia coli (EPEC) infection inhibits butyrate uptake in Caco-2 cells. Caco-2 cells at 12-14 days postplating were serum starved overnight and then infected for 60 min with EPEC or nonpathogenic E. coli (HS4). butyrate uptake was subsequently measured as described in MATERIALS AND METHODS. Results represent means ± SE of 5 separate experiments performed in triplicate. * P < 0.05 compared with control.
Time course of EPEC inhibition of butyrate uptake. The time course of EPEC-mediated inhibition of butyrate uptake was determined by infecting Caco-2 monolayers with EPEC for 15, 30, 60, 90, and 120 min. As shown in Fig. 2, at earlier time points of infection (15 and 30 min), butyrate uptake was not significantly decreased, whereas 70% inhibition of butyrate uptake occurred by 60 min postinfection and persisted for at least 120 min. Therefore, all subsequent experiments were performed at 60 min postinfection.
Fig. 2. Time course of EPEC inhibition of butyrate uptake. Caco-2 monolayers were infected with EPEC for 15, 30, 60, 90, and 120 min, and butyrate uptake was measured. Results represent means ± SE of 3 independent experiments performed in triplicate.
EPEC infection decreases the maximal velocity of butyrate uptake without altering the Michaelis constant. The kinetics of butyrate uptake by EPEC-infected and uninfected Caco-2 cells were measured using increasing substrate concentrations in the range of 0.5-15 mM. As shown in Fig. 3, EPEC infection significantly decreased the activity of butyrate uptake at all concentrations examined. An analysis of the Lineweaver-Burke plot of this data showed that maximal velocity ( V max ) of butyrate uptake in EPEC-infected cells compared with uninfected controls was reduced significantly ( V max : in nmol·mg protein -1 ·5 min -1 : 15.9 ± 0.1 for control vs. 9.1 ± 0.1 in response to EPEC infection). In contrast, the apparent Michaelis constant ( K m ) did not change significantly (control 2.52 ± 0.04 mM vs. EPEC infected 2.71 ± 0.02 mM ).
Fig. 3. Effect of EPEC infection on the kinetics of butyrate uptake in Caco-2 cells. Caco-2 cells were infected with wild-type EPEC for 60 min, and butyrate uptake was determined in the presence of increasing concentrations (0.5-15 mM) of butyrate. The experiment was performed on 3 separate occasions of EPEC infection. A Michaelis-Menten plot from a representative experiment is shown.
TTSS is required to inhibit butyrate uptake. The role of various EPEC virulence proteins on the EPEC-induced decrease in butyrate uptake by Caco-2 cells was investigated using specific mutant strains. First, involvement of the TTSS was studied using an escN mutant strain. The product of this gene is the putative ATPase that drives type III secretion of various virulence proteins ( 9 ). As shown in Fig. 4, there was no inhibition of butyrate uptake by Caco-2 cells when infected with the escN mutant strain, in contrast to significant inhibition by wild-type EPEC. These results reveal that a functional TTSS is required for EPEC to inhibit butyrate uptake by Caco-2 cells.
Fig. 4. A functional type III secretion system (TTSS) is required for EPEC-mediated inhibition of butyrate uptake. Caco-2 cells were infected with EPEC or escN mutant strain for 60 min, and then butyrate uptake was measured. Results represent means ± SE of 3 independent experiments performed in triplicate. * P < 0.05 compared with control.
EPEC secreted proteins are involved in modulating butyrate uptake. We further analyzed the role of the TTSS in EPEC-modulated butyrate uptake by infecting cells with either espA, espB, or espD mutant strains. Each of these virulence genes encodes a structural component of the TTSS; therefore, mutation of any one of them renders TTSS ineffective ( 22 ). Figure 5 shows that infection of cells with these mutants had no effect on butyrate uptake compared with uninfected controls. Infection with wild-type EPEC, as expected, resulted in a marked decline in butyrate uptake. These results indicate that the structural components of the translocation apparatus themselves or the secreted effector molecules are required for the observed effect of EPEC on butyrate uptake.
Fig. 5. EPEC-secreted proteins EspA, EspB, and EspD are required for EPEC inhibition of butyrate uptake. Caco-2 cells were infected with EPEC or one of the mutant strains espA, espB, or espD for 60 min, and butyrate uptake was determined. Results represent means ± SE of 4 separate experiments performed in triplicate. * P < 0.05 compared with control.
Effector proteins EspF, EspG, EspH, and Map are not involved. EPEC infection results in the delivery of a number of effector proteins in the host cytosol. The role of these effector molecules in modulating butyrate uptake was also studied. Figure 6 shows that mutation of espF, – G, and – H, map, or of a double mutation of espG and its homolog orf3 had no impact on the inhibition of butyrate uptake caused by wild-type EPEC. These results suggest that these effector proteins are not involved in EPEC’s effect on butyrate uptake.
Fig. 6. EspF, EspG, EspH, and Map are not required for inhibition of butyrate uptake. Caco-2 cells were infected with EPEC, espF, espG, espH, map, or espG/orf3 double mutants for 60 min, and butyrate uptake was then measured. Results represent means ± SE of 3 separate experiments in triplicate. * P < 0.05 compared with control.
Membrane expression of MCT1 is decreased by EPEC infection. It has been previously shown by us and others ( 11, 28 ) that MCT1 is involved in the uptake of butyrate by Caco-2 cells. Therefore, to examine the effect of EPEC infection on MCT1 protein, cell surface proteins from control and infected cells were biotinylated and pulled down from the cell lysate by avidin, and separated proteins were probed with an anti-MCT1 antibody. As shown in Fig. 7 A, surface expression of MCT1 in infected cells was decreased considerably compared with uninfected controls or those infected with nonpathogenic E. coli (HS4), whereas levels of total cellular MCT1 remained constant in all groups. Densitometric scanning 50% decrease in the amount of surface MCT1 compared with total MCT1 in EPEC-infected cells ( Fig. 7 B ).
Fig. 7. A : surface expression of monocarboxylate transporter 1 (MCT1) is reduced in response to EPEC infection. Caco-2 cells were infected with EPEC or nonpathogenic E. coli ( HS4 ) for 60 min. Cell surface proteins were biotinylated as described in MATERIALS AND METHODS. Biotinylated proteins were pulled down from equal aliquots of cell lysates (protein contents normalized to 2.5 mg/ml) using equal amounts of streptavidin agarose. SDS-PAGE of the biotinylated proteins or the total proteins in cell lysates were probed with anti-MCT1 antibody. The blot is representative of 3 separate experiments. B : densitometric scanning of the relative band intensities and ratio of surface MCT1 to total MCT1. Values are means ± SE of 3 separate experiments. * P < 0.05 compared with control.
DISCUSSION
Absorption of SCFAs is important for colonocyte health and metabolism, epithelial integrity, as well as colonic fluid and electrolyte balance. Butyrate, a key SCFA, is known to have multiple regulatory roles in the mammalian colon, including stimulation of fluid and electrolyte absorption ( 24 ) by increasing electroneutral NaCl absorption ( 3 ) and inhibiting Cl – secretion ( 27 ). Recent studies ( 19 ) have also demonstrated that butyrate stimulates promoter activity and expression of the apical NHE3 in the human adenocarcinoma cell line Caco-2. Butyrate has also been implicated in suppressing mucosal inflammation ( 16 ). Decreased production or availability of butyrate has been shown to result in chronic inflammation and acute diarrhea ( 6 ). However, to date, the effects of enteric pathogens on the absorption of SCFAs have not been examined. EPEC is a human enteric pathogen infecting primarily infants and young children. The specific mechanisms by which EPEC causes early diarrhea in infected hosts, however, remain unclear. Diarrhea results from either increased secretion, impaired absorption, or both. EPEC infection has intact been shown to decrease secretagogue-induced Cl – secretion ( 15 ). We have also shown that EPEC infection increases NHE2 activity, whereas the activity of NHE3, the predominant Na + -absorbing isoform, is inhibited ( 14 ). Cl – /OH – exchange activity was also shown to be inhibited in Caco-2 cells infected with EPEC ( 13 ). We have also reported that EPEC infection induces inflammation ( 29 ) and disrupts the structure and barrier function of tight junctions after prolonged infections ( 26 ). These findings suggest that the mechanism(s) of EPEC-induced diarrhea are multifactorial and may also involve modulation of SCFA uptake. Caco-2 cell monolayers were selected to investigate this question, since previous studies from our laboratory ( 11 ) demonstrated that this cell line was a suitable in vitro model to study butyrate uptake. The data presented here suggest that EPEC infection of confluent differentiated Caco-2 monolayers results in a significant decrease in butyrate uptake. This effect was specific, since infection with nonpathogenic E. coli (HS4) had no effect on butyrate uptake. Time course studies of EPEC inhibition of butyrate uptake showed effective inhibition as early as 60 min postinfection that persisted until 120 min.
EPEC is noninvasive and does not produce toxins. Instead, it employs the TTSS to deliver virulence factors directly in the host cells ( 32 ). Several mutational studies have enabled identification of individual components of the TTSS. Requirement of an intact TTSS for EPEC to inhibit butyrate uptake is supported by the fact that mutation of the putative ATPase (EscN) blocked the inhibitory effect of EPEC infection. This mutant has also been shown to be ineffective in modulating Na + ( 14 ) and Cl – ( 10 ) uptake by Caco-2 cells. Mutant strains carrying mutations in espA, espB, or espD, all of which interfere with type III secretion, also failed to inhibit butyrate uptake in infected Caco-2 cells. EspA forms a filamentous organelle on the bacterial surface that is postulated to act as a channel for the type III system to deliver proteins to the host cell ( 9 ). EspB and EspD are translocated to the host cell membrane and together are thought to form the translocation pore. EspB also has been reported to be present in the cytoplasm; however, its function here is not known ( 9 ).
The EPEC-secreted effector molecules have previously been reported to alter host cell functions via different mechanisms. For example, EspF is known to disrupt tight junctions ( 23 ); EspG, and its homolog Orf3, both disrupt microtubules and produce subtle alterations in barrier functions ( 8, 30 ); EspH is known to alter pedestal morphology and filopodia formation ( 31 ), whereas Map has been reported to alter mitochondrial membrane potential ( 18 ). However, our current studies suggested that the secreted effector molecules EspF, EspG, EspH, and Map were not involved in mediating the observed decrease in butyrate uptake in response to EPEC infection, since these mutants behaved similar to wild-type EPEC in inhibiting butyrate uptake. Orf3, the espG homolog, is encoded in the EPEC genome in a locus distinct from the LEE. Because both effectors (EspG and Orf3) are known to induce disruption of microtubule networks beneath adherent bacteria ( 21 ), we used a double mutant of espG / orf3 to infect cells and observe the effects on butyrate uptake. There was again a significant inhibition of butyrate uptake, similar to that caused by wild-type EPEC, suggesting that these effector molecules are not involved in EPEC-mediated inhibition of butyrate uptake. However, the inability of nonpathogenic E. coli or type III secretion mutants to inhibit butyrate uptake seems to indicate that the observed effects are related to pathogenicity.
Our kinetic studies suggested that EPEC inhibited butyrate uptake via changes in the value of V max without significantly altering the apparent K m, indicating a decrease in the expression of the butyrate transporter on the plasma membrane. This was indeed found to be the case by performing biotinylation studies to quantitate the amount of surface MCT1, the butyrate transporter in Caco-2 cells. Although the surface expression of MCT1 was considerably lower in cells infected with EPEC compared with nonpathogenic E. coli, or uninfected control cells, total cellular MCT1 was the same in all the groups. These studies demonstrate the regulation of butyrate uptake either by retrieval of MCT1 from the apical plasma membrane or via reduced targeting to the apical membrane in response to EPEC infection. In this regard, previous studies of Buyse et. al. ( 5 ) also suggested that MCT1 is regulated by luminal leptin by altering translocation of MCT1 to the apical plasma membrane.
The effects of pathogenic organisms on fluid and electrolyte secretion by host intestinal epithelial cells have been well documented. Various enteric pathogens elicit a Cl – secretory response. The studies from our laboratory showed profound early effects of EPEC infection on intestinal epithelial absorption, an aspect that has not been studied previously. The observed effects of EPEC infection on butyrate uptake reported here and previously published results from our laboratory on Na + /H + and Cl – /OH – exchange activities strongly suggest that EPEC infection has profound effects on the host intestinal absorptive ion transport processes. We speculate that decreased butyrate availability caused by EPEC infection might compromise colonic epithelial integrity, resulting in inflammation of the epithelium, and also might contribute to EPEC-mediated diarrhea by inhibiting ion absorption in the colon. Our results demonstrate that EPEC requires a TTSS to inhibit butyrate uptake via regulation of MCT1. In lieu of the important role of SCFA in stimulating human colonic electrolyte absorption and being the key nutrient in the colon, our studies not only add to increased understanding of the mechanisms regulating human intestinal SCFA absorption but also suggest the potential role of inhibition of this key ion as an important contributory factor in the pathophysiology of EPEC-associated diarrhea.
GRANTS
These studies were supported by the Department of Veterans Affairs and the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-54016 (P. K. Dudeja), DK-68324 (P. K. Dudeja), DK-33349 (K. Ramaswamy), DK-67990 (K. Ramaswamy), and DK-50694 (G. Hecht).
【参考文献】
Akhter S, Kovbasnjuk O, Li X, Covet M, Noel J, Arpin M, Hubbard AL, and Donowitz M. Na + /H + exchanger 3 is in large complexes in the center of the apical surface of proximal tubule-derived OK cells. Am J Physiol Cell Physiol 283: C927-C940, 2002.
Alrefai WA, Tyagi S, Gill R, Saksena S, Hadjiagapiou C, Mansour F, Ramaswamy K, and Dudeja PK. Regulation of butyrate uptake in Caco2 cells by phorbol 12-myristate 13-acetate. Am J Physiol Gastrointest Liver Physiol 286: G197-G203, 2004.
Binder HJ and Mehta P. Short chain fatty acids stimulate Na and Cl absorption in vitro in the rat distal colon. Gastroenterology 96: 989-996, 1989.
Bradford M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254, 1976.
Buyse M, Sitaraman SV, Liu X, Bado A, and Merlin D. Luminal leptin enhances CD147/MCT1 mediated uptake of butyrate in the human intestinal cell line Caco2-BBE. J Biol Chem 277: 28182-28190, 2002.
Cook SI and Sellin JH. Review article: short chain fatty acids in health and disease. Pharmacol Ther 12: 499-507, 1998.
Donnenberg MS, Yu J, and Kaper JB. A second chromosomal gene necessary for intimate attachment of enteropathogenic E. coli to epithelial cells. J Bacteriol 175: 4670-4680, 1993.
Elliott SJ, Krejany EO, Mellies JL, Robins-Browne RM, Sasakawa C, and Kaper JB. EspG, a novel type III system-secreted protein from enteropathogenic E. coli with similarities to VirA of Shigella flexneri. Infect Immun 69: 4027-4033, 2001.
Gauthier A, Puente JL, and Finlay BB. Secretion of the enteropathogenic E. coli type III secretion system requires components of the type III apparatus for assembly and localization. Infect Immun 71: 3310-3319, 2003.
Gill Ravinder K, Hodges K, Borthakur A, Saksena S, Ramaswamy K, Hecht GA, and Dudeja PK. Role of enteropathogenic E. coli virulence genes in inhibition of apical Cl – /OH – exchange activity in Caco-2 cells (Abstract). Gastroenterology 126: A-95, 2004.
Hadjiagapiou C, Schmidt L, Dudeja PK, Lyden TJ, and Ramaswamy K. Mechanism(s) of butyrate uptake in Caco2 cells: Role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol 279: G775-G780, 2000.
Harig JM, Ng EK, Dudeja PK, Brasitus TA, and Ramaswamy K. Transport of n-butyrate into human colonic luminal membrane vesicles. Am J Physiol Gastrointest Liver Physiol 271: G415-G422, 1996.
Hecht G, Gill R, Saksena S, Tyagi S, Hodges K, Ramaswamy K, and Dudeja PK. Enteropathogenic E. coli inhibits Cl – /OH – exchange activity in Caco2 cells (Abstract). Gastroenterology 124: A482, 2003.
Hecht G, Hodges K, Gill R, Kear F, Tyagi S, Malakooti J, Ramaswamy K, and Dudeja PK. Differential regulation of Na + H + exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 287: G370-G378, 2004.
Hecht G and Koutsouris A. Enteropathogenic E coli attenuates secretagogue-induced net intestinal ion transport but not Cl- secretion. Am J Physiol Gastrointest Liver Physiol 276: G781-G788, 1999.
Inan MS, Rasoulpour RJ, Yin L, Habbard AK, Rosenberg DW, and Giardena C. The luminal short chain fatty acid butyrate modulate NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 118: 724-734, 2000.
Jarvis KG, Giron JA, Jerse AE, McDaniel TK, Donnenberg MS, and Kaper JB. Enteropathogenic E. coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA 92: 7996-8000, 1995.
Kenny B and Jepson M. Targeting of an EPEC effector protein to host mitochondria. Cell Microbiol 2: 579-590, 2000.
Kiela PR, Hines ER, Collins JF, and Ghisan FK. Regulation of the rat NHE3 gene promoter by sodium butyrate. Am J Physiol Gastrointest Liver Physiol 281: G947-G956, 2001.
Lai LC, Wainwright LA, Stone KD, and Donnenberg MS. A third secreted protein that is encoded by the enteropathogenic E. coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun 65: 2211-2217, 1997.
Matsuzawa T, Kuwae A, Yoshida S, sasakawa C, and Abe A. Enteropathogenic E. coli activates the RhoA signalling pathway via the stimulation of GEF-H1. EMBO J 23: 3570-3582, 2004.
McDaniel TK and Kaper JB. A cloned pathogenicity island from enteropathogenic E. coli confers the attaching and effacing phenotype on E coli k-12. Mol Microbiol 23: 399-407, 1997.
McNamara BP, Koutsouris A, O’conell CB, Nougayrede JP, Donnenberg MS, and Hecht G. Translocated EspF protein from enteropathogenic E. coli disrupts host intestinal barrier function. J Clin Invest 107:621-629, 2001.
Montrose MH and Kere J. Anion absorption in the intestine: anion transporters, short chain fatty acids, and role of the DRA gene product. Curr Top Membr Transp 50: 301-928, 2001.
Musch MW, Bookstein C, Xie Y, Sellin JH, and Chang EB. Short chain fatty acids increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. Am J Physiol Gastrointest Liver Physiol 280: G687-G693, 2001.
Muza-Moons MM, Schneeberger EE, and Hecht GA. Enteropathogenic E. coli infection leads to appearance of aberrant tight junction strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol 6: 783-793, 2004.
Resta-Lenert S, Trung F, Barrett KE, and Eckmann L. Inhibition of epithelial chloride secretion by butyrate: role of reduced adenylyl cyclase expression and activity. Am J Physiol Cell Physiol 281: C1837-C1849, 2001.
Ritzhaupt A, Wood IS, Ellis A, Hosie KB, and Shirazi-Beechey SP. Identification and characterization of a monocarboxylate transporter. J Physiol 513: 719-732, 1998.
Savkovic SV, Koutsouris A, and Hecht A. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic E. coli. Am J Physiol Cell Physiol 273: C1160-C1167, 1997.
Tomson FL, Viswanathan VK, Kanack KJ, Kanteti RP, Straub KV, Menet M, Kaper JB, and Hecht G. Enteropathogenic E. coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier. Mol Microbiol 56: 447-464, 2005.
Tu X, Nisan I, Yona C, Hanski E, and Rosenshine I. EspH, a new cytoskeleton-modulating effector of enterohaemorrhagic and enteropathogenic E. coli. Mol Microbiol 47: 595-606, 2003.
Vallance BA and Finlay BB. Exploitation of host cells by enteropathogenic E. coli. Proc Natl Acad Sci USA 97: 8799-8806, 2000.
Dietary Sodium Butyrate Decreases Postweaning Diarrhea by Modulating Intestinal Permeability and Changing the Bacterial Communities in Weaned Piglets
http://jn.nutrition.org/content/145/12/2774.full?cited-by=yes&legid=nutrition;145/12/2774
http://jn.nutrition.org/content/145/12/2774.full.pdf+html
Dietary Sodium Butyrate Decreases Postweaning Diarrhea by Modulating Intestinal Permeability and Changing the Bacterial Communities in Weaned Piglets1,2,3
- Chang Huang4,7,
- Peixia Song4,7,
- Peixin Fan4,
- Chengli Hou4,
- Phil Thacker5, and
- Xi Ma4,6,*
+Author Affiliations
- ↵*To whom correspondence should be addressed. E-mail: maxi@cau.edu.cn or xi.ma@utsouthwestern.edu.
Abstract
Background: The vast majority of substances used as alternatives to antibiotics produce inconsistent results and rarely equal the effectiveness of in-feed antibiotics.
Objective: This study evaluated the effects of the combined use of sodium butyrate (SB) and reduced antibiotics in a piglet diet in promoting performance and to control weaning diarrhea.
Methods: Piglets weaned at 28 d were randomly assigned to a corn-soybean meal control ration [negative control (NC)]; a similar ration with 50 mg kitasamycin/kg, 20 mg colistin sulfate/kg, and 1000 mg encapsulated SB/kg [reduced antibiotics + SB (ASB)]; or to a ration with 100 mg kitasamycin/kg and 40 mg colistin sulfate/kg [positive control (PC)] for 28 d. Performance, diarrhea incidence, intestinal permeability, and changes in the bacterial communities in the ileum and colon were determined.
Results: Weight gain and the ratio of weight gain to feed intake were significantly greater in the ASB and PC piglets than in the NC piglets (P < 0.05). Diarrhea incidence was lower in the ASB and PC piglets than in the NC piglets (P < 0.05). Urinary lactulose to mannitol ratios were 25% and 30% lower, respectively, whereas jejunal and colonic occludin protein expressions were significantly greater in the ASB and PC piglets compared with the NC piglets (P < 0.05). In the intestinal mucosa, malondialdehyde was lower in the ASB and PC piglets (by 42% and 43%, respectively), whereas tumor necrosis factor α (TNF-α) was 63% lower in the ASB piglets and 59% lower in the PC piglets compared with the NC piglets (P < 0.05). 16S ribosomal RNA gene sequence analysis revealed a higher colonic Shannon index and a lower colonic Simpson index in the ASB and PC piglets than in the NC piglets. In addition, the ASB and PC treatments caused a striking decrease in Lactobacillaceae and a noticeable increase in Clostridiaceae in the ileal and colonic lumen, as well as increases in Ruminococcaceae, Lachnospiraceae, and Bacteroidetes in the colonic lumen.
Conclusion: Collectively, our results support an important role for SB in improving performance and decreasing diarrhea incidence in weaned piglets by modulation of intestinal permeability and the bacterial communities in the ileum and colon.
Keywords:
- sodium butyrate
- antibiotic alternative
- performance
- intestinal permeability
- bacterial communities
- piglet
Introduction
The period after weaning is characterized by a high incidence of intestinal disturbances with diarrhea and decreased performance in piglets (1). Subtherapeutic doses of antibiotics have been proven to increase growth rate, improve feed utilization, and reduce the incidence of postweaning diarrhea (2). However, indiscriminate use of antibiotics has led to an increasing number of antibiotic-resistant pathogens as well as public concern with regard to their cross-transfer to humans (3). As a result, the use of antibiotics in diets fed to livestock has been banned in the European Union since 2006. Many materials have been investigated as alternatives (4), among which some organic acids and their salts incorporated in diets are known to be helpful in overcoming the postweaning syndrome (3).
Butyrate, an SCFA, is physiologically produced by large bowel microbial fermentation of dietary carbohydrates in mammals (5). It is quickly absorbed and can be utilized as a major energy source by epithelial cells in the terminal ileum and large intestine (6), as well as stimulating growth of the small intestinal epithelium (5). The effects of butyrate on carcinogenesis (5), inflammation (7), oxidative stress (8), and intestinal barrier function (9) have been described, particularly its activities in promoting gut health both in vitro (10) and in vivo (11).
Because it is easier to handle in the feed-manufacturing process, butyrate is often used in the form of sodium salt instead of free acid (5). Accumulated studies have been conducted with regard to the effects of sodium butyrate (SB)8 on performance in weaned piglets, including feed intake, weight gain, and feed efficiency. One trial by Piva et al. (12) showed that SB supplementation at 800 mg/kg increased weight gain and feed intake in piglets in the first 2 wk after weaning (P < 0.05). In another feeding trial, weaned piglets fed 1000-mg-SB/kg diets showed improved performance compared with those fed a control diet or 500 mg SB/kg (P < 0.05) (13). However, inconsistent results were obtained in other studies. Performance did not differ between piglets fed a basal diet with SB at 0, 1000, 2000, or 4000 mg/kg in an experiment by Biagi et al. (14). Weber and Kerr (15) reported that there was no effect of dietary SB (500, 1000, 2000, and 4000 mg/kg) on overall feed efficiency. In another trial, dietary SB (1000 mg/kg) significantly decreased diarrhea incidence in weaned piglets (P < 0.05) without improvements in weight gain, feed intake, or feed efficiency (16). A conclusion can be drawn that SB does not consistently provide growth-promoting effects to weaned piglets. Therefore, it would appear that SB cannot be used as the sole alternative to antibiotics in piglet feeding.
Although many other countries are expected to introduce an antibiotic ban in diets fed to livestock, antibiotics are still extensively used in many areas of the world. The entire withdrawal of antibiotics in piglet diets would likely result in considerable production loss in these areas because the vast majority of antibiotic substitutes tested produce inconsistent results and rarely equal the effectiveness of antibiotics (4). An efficient strategy may be to use a partial substitution of antibiotics with other growth-promoting substances, as suggested by a previous study in which supplemental acidifiers appeared to act synergistically with avilamycin (17). However, to our knowledge, few studies have focused on the application of SB inclusion, combined with decreased antibiotics, in piglet feeding.
In the present study, we evaluated the effects of the combined use of SB and reduced antibiotics in promoting performance and to control postweaning diarrhea in piglets. Furthermore, to probe into the possible mechanisms for these effects, some variables with regard to intestinal barrier function and changes in the bacterial communities in the ileum and colon were determined.
Methods
Piglets, diets, and experimental protocol.
All procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of China Agricultural University. The heaviest 90 crossbred (Duroc × Landrace × Large White) weaned piglets (weaned at 28 d) were selected from a pool of 30 litters. After a 3-d adaptation period during which all piglets were fed the same base diet, the pigs (10.24 ± 1.90 kg body weight) were assigned to 1 of 3 treatments (n = 30) that were homogenous for weight and sex. A basal diet was formulated to meet the nutrient requirements of the pigs according to the NRC (18) (Supplemental Table 1). Dietary treatments included a corn-soybean meal control ration without antibiotics [negative control (NC)]; a similar ration with 50 mg kitasamycin/kg, 20 mg colistin sulfate/kg, and 1000 mg encapsulated SB/kg [Lideshi, Inc.; reduced antibiotics + SB (ASB)]; and a ration with 100 mg kitasamycin/kg and 40 mg colistin sulfate/kg [positive control (PC)]. The encapsulated structure in SB was used to deter prompt absorption and metabolism of butyrate in the duodenum and jejunum, which ensured considerable release and absorption of butyrate in the lower intestine portion (19).
During the 28-d experiment, pigs were fed their respective diets and allowed ad libitum access to feed and water. The piglets were individually weighed on day 28, and feed consumption per pen was recorded weekly. The amount of feed wasted was recorded daily.
Fecal consistency was visually assessed at 0900 and 1600 h each day by observers who were blind to treatments with the use of a modification of the method described by Ma et al. (20). Fresh excreta were graded by using the following scale: 0 = solid, 1 = semisolid, 2 = semiliquid, and 3 = liquid. The occurrence of diarrhea was defined as production of grade 2 or 3 feces for 2 continuous days.
Small intestinal permeability.
Small intestinal permeability was assessed on day 28 before weighing by using the lactulose to mannitol differential absorption test (21). Briefly, urine samples of piglets per treatment (n = 6) were collected after 6 h of feed deprivation for baseline urinary sugar measurement. After the samples were obtained, 5 mL lactulose (0.4 g/mL; Sigma) and 5 mL of mannitol (0.2 g/L; Sigma) were administered intragastrically to the piglets. Piglets were feed-deprived for the 6-h study period but were allowed to drink water after 30 min. Urinary lactulose and mannitol concentrations were determined by an enzymatic technique (22). Mannitol excretion was corrected by subtraction of baseline values, and the lactulose to mannitol excretion ratio was calculated as an index of intestinal permeability.
Sample collection.
On day 28, 6 blood samples per treatment were harvested from the jugular vein by using tubes without anticoagulant (Becton Dickinson). Blood samples were allowed to clot at room temperature for 20 min and centrifuged at 1610 × g for 10 min at 4°C. Serum was then removed and stored at −20°C until assay. Intestinal segments (duodenum, jejunum, ileum, and colon); mucosa in the duodenum, jejunum, ileum, and colon; as well as chyme from the ileum and colon were obtained and stored at −80°C until further assay (23).
Morphologic evaluations.
Villus height and crypt depth in the duodenum, jejunum, and ileum were determined as previously described (23). Briefly, these segments were fixed in 4% paraformaldehyde for 24 h and then embedded in paraffin wax. Sections of 4 μm were cut and stained with hematoxylin and eosin. Measurements for villus height and crypt depth were taken by using the Axioskop-2 microscope (Olympus) and the Image Processing System (Visitron Systems).
Protein extraction and immunoblot analysis.
The expression of the protein occludin was determined due to its crucial roles in tight junction structure and paracellular permeability (24). The total protein amount contained in the jejunal and colonic tissue samples was extracted according to the method described by a ProteoJET Total Protein Extraction Kit (Fermentas). A Bicinchoninic Acid Protein Assay Kit (Applygen Technologies) was used to determine the protein concentration. Equal amounts of protein extracts (20 μg) were fractionated on 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories). The membranes were blocked with a 5% skimmed-milk solution at room temperature for 2 h and then incubated with diluted antibodies against occludin (1:200; Santa Cruz) and GAPDH (1:20,000; Sigma). After incubation with HRP-conjugated secondary antibody, signals were visualized by the Odyssey Infrared Imaging System (LI-COR Biosciences). Blot analysis was carried out at 6 replicates/treatment by Quantity One software (BioRad Laboratories), with subsequent calculation of the ratio between the band intensities of occludin and GAPDH.
Antioxidant variables and immune indexes.
Serum samples were thawed and thoroughly mixed immediately before testing. Equal amounts of mucosa in different intestinal segments from the same piglet were blended to form a single sample. The blended samples (n = 6/treatment) were homogenized in PBS (10 mM; pH 7.4) and centrifuged at 3000 × g for 10 min at 4°C. The supernatant was stored at −80°C until further assays.
Antioxidant variables, including superoxide dismutase, glutathione peroxidase, glutathione, malonaldehyde, as well as the cytokines complement 3, IL-1β, IL-2, IFN-γ, and TNF-α in serum and intestinal mucosa were all determined by using assay kits according to the manufacturer’s instructions. All of the assay kits were purchased from the Nanjing Jiancheng Bioengineering Institute.
Composition and diversity of the bacterial communities.
DNA in each ileal and colonic chyme sample was extracted according to the manufacturer’s protocol (Bocai Biology). The V3 and V4 regions of the 16S ribosomal DNA gene were chosen for PCR. The primers were 338F (5′ACTCCTACGGGAGGCAGCA-3′) and 806R (5′GGACTACHVGGGTWTCTAAT-3′). The procedure to obtain amplicons was previously described, with modifications (25). The products were examined on a 2% (wt:vol) agarose gel. The amplicons from 6 replicates/treatment were blended in equimolar ratios on the basis of concentration. The blended samples were sent out for MiSeq Next-Generation Sequencing System on an Illumina MiSeq PE300 platform at the Majorbio Bio-Pharm Technology Company. Only sequences >50 bp were used for phylotype analysis at the 0.03 operational taxonomic unit level.
Statistical analysis.
Differences in diarrhea incidence between the 3 treatments were tested by the chi-square contingency test. All other data were analyzed by using SAS version 9.1. The results are presented as means ± SEMs. One-factor ANOVA followed by Tukey’s multiple-range tests were used for equal variances. Kruskal-Wallis 1-factor ANOVA was performed to compare means with unequal variances. For all statistical analyses, P values <0.05 were considered significant.
Results
Performance and diarrhea incidence.
Compared with the NC piglets, weight gain was significantly greater in the ASB and PC piglets (P < 0.05) with feed intake unchanged, resulting in an improved gain to feed ratio in the ASB and PC piglets (P < 0.05) (Table 1). Diarrhea incidence was significantly lower in the ASB and PC piglets than in the NC piglets (P < 0.05) (Table 1). There were no significant differences in performance or diarrhea incidence between the ASB and PC piglets.
View this table:
TABLE 1
Performance and diarrhea incidence in weaned piglets fed corn-soybean meal without antibiotics (NC), corn-soybean meal with reduced antibiotics and sodium butyrate (ASB), or corn-soybean meal with antibiotics (PC) for 28 d1
Small intestinal morphology.
Villus height in the jejunum and crypt depth in the duodenum and jejunum were lower in the ASB piglets than in the NC and PC piglets (P < 0.05). The ASB piglets had lower crypt depth in the ileum than did the NC piglets (P < 0.05), whereas the value for the PC piglets was intermediate to values for the NC and ASB piglets (Table 2).
View this table:
TABLE 2
Small intestinal morphology of weaned piglets fed corn-soybean meal without antibiotics (NC), corn-soybean meal with reduced antibiotics and sodium butyrate (ASB), or corn-soybean meal with antibiotics (PC) for 28 d1
Villus height in the duodenum and ileum was greater in the PC piglets than in the NC and ASB piglets (P < 0.05). Villus height to crypt depth ratio in the ileum in the PC piglets was also greater than in the NC piglets (P < 0.05), whereas the ratio for the ASB piglets was intermediate to that for the NC and PC piglets (Table 2).
Small intestinal permeability and occludin abundance.
Small intestinal permeability was lower in the ASB and PC piglets as indicated by the decreased urinary lactulose to mannitol ratios compared with the NC piglets (P < 0.05) (Figure 1). The expression of the intestinal tight junction protein occludin in the jejunum and colon was significantly higher in the ASB and PC piglets than in the NC piglets (P < 0.05) (Figure 2). These variables in the ASB piglets were comparable to those in the PC piglets.
FIGURE 1
Small intestinal permeability in weaned piglets fed corn-soybean meal without antibiotics (NC treatment), corn-soybean meal with reduced antibiotics and encapsulated sodium butyrate (ASB treatment), or corn-soybean meal with antibiotics (PC treatment) for 28 d. Values are means ± SEMs, n = 6. Labeled means without a common letter differ, P < 0.05. ASB, reduced antibiotics + sodium butyrate; NC, negative control; PC, positive control.
FIGURE 2
Immunoblot analysis of occludin protein abundance in the jejunal (A) and colonic (B) tissue of weaned piglets fed corn-soybean meal without antibiotics (NC treatment), corn-soybean meal with reduced antibiotics and encapsulated sodium butyrate (ASB treatment), or corn-soybean meal with antibiotics (PC treatment) for 28 d. Representative Western blots are shown. Values are means ± SEMs, n = 6. Labeled means without a common letter differ, P < 0.05. ASB, reduced antibiotics + sodium butyrate; NC, negative control; PC, positive control.
Antioxidant variables.
The serum content of superoxide dismutase in the ASB piglets was significantly higher compared with that in the PC piglets (P < 0.05), whereas the value for the NC piglets was at an intermediate level. Activities of glutathione peroxidase in the intestinal mucosa were increased in the ASB piglets compared with the NC and PC piglets (P < 0.05). The ASB piglets had decreased malondialdehyde in the serum compared with the NC piglets (P < 0.05), whereas the value for the PC piglets was intermediate to that of the NC and ASB treatments. The malondialdehyde concentration in the intestinal mucosa in the ASB piglets was comparable to that in the PC piglets, both of which were significantly lower than in the NC piglets (P < 0.05) (Table 3).
View this table:
TABLE 3
Antioxidant variables in serum and intestinal mucosa of weaned piglets fed corn-soybean meal without antibiotics (NC), corn-soybean meal with reduced antibiotics and sodium butyrate (ASB), or corn-soybean meal with antibiotics (PC) for 28 d1
Immune indexes.
Serum IFN-γ was higher in the ASB piglets compared with the NC piglets (P < 0.05), whereas the value for the PC piglets was at an intermediate level. The PC piglets had a lower concentration of TNF-α in serum than did the NC piglets (P < 0.05), whereas the value for the ASB piglets was intermediate to those for the NC and PC piglets. In the intestinal mucosa, TNF-α concentrations in the ASB and PC piglets were lower than in the NC piglets (P < 0.05), although no significant difference was observed between the ASB and PC piglets (Table 4).
View this table:
TABLE 4
Immune indexes in serum and intestinal mucosa of weaned piglets fed corn-soybean meal without antibiotics (NC), corn-soybean meal with reduced antibiotics and sodium butyrate (ASB), or corn-soybean meal with antibiotics (PC) for 28 d1
Bacterial composition and diversity.
The ileal lumen harbored decreased bacterial diversity with the ASB and PC treatments, as indicated by the decreased Shannon index and increased Simpson index, whereas the bacterial community in the colonic lumen became more diverse (Table 5). A noticeable increase was observed in Bacteroidetes by the ASB and PC treatments, from 0.7% in the NC piglets to 3.4% in the ASB piglets and 10.3% in the PC piglets (data not shown). The majority of classifiable sequences belonged to Lactobacillaceae (63.8%), Streptococcaceae (18.3%), Pasteurellaceae (7.2%), and Enterobacteriaceae (6.3%) in the ileal lumen, whereas the colonic lumen was dominated by Lactobacillaceae (64.6%), Streptococcaceae (10.1%), Ruminococcaceae (7.7%), Lachnospiraceae (6.0%), and Neisseriaceae (4.0%) in the NC piglets (Figure 3). The decreases in Lactobacillaceae abundance with exposure to the ASB and PC were striking: from 63.8% in the ileal lumen of the NC piglets to 6.8% with the ASB treatment and 8.7% with the PC treatment; the values for the colonic lumen were 64.6%, 44.4%, and 4.4%, respectively. Nonabundant Clostridiaceae became dominant in the ileal lumen, with large increases from 0.3% in the NC piglets to 83.2% in the ASB piglets and 35.0% in the PC piglets (Figure 3). In addition, noticeable increases in Clostridiaceae abundance were also observed in the colonic lumen with the ASB (1.6%) and PC (10.9%) treatments compared with the NC piglets (0.3%) (Figure 3). In addition, certain bacterial families decreased with the ASB and PC treatments, such as Pasteurellaceae and Enterobacteriaceae in the ileal lumen, whereas Ruminococcaceae and Lachnospiraceae were increased in the colonic lumen with the ASB and PC treatments (Figure 3).
View this table:
TABLE 5
Bacterial community diversity in the ileal and colonic chyme of weaned piglets fed corn-soybean meal without antibiotics (NC), corn-soybean meal with reduced antibiotics and sodium butyrate (ASB), or corn-soybean meal with antibiotics (PC) for 28 d1
FIGURE 3
Family-level distribution of luminal bacteria in the ileal and colonic chyme of weaned piglets fed corn-soybean meal without antibiotics (NC treatment), corn-soybean meal with reduced antibiotics and encapsulated sodium butyrate (ASB treatment), or corn-soybean meal with antibiotics (PC treatment) for 28 d. ASB, reduced antibiotics + sodium butyrate; NC, negative control; PC, positive control.
Discussion
Kitasamycin is an antibiotic growth promoter that exhibits activity mainly against gram-positive micro-organisms (26), whereas colistin sulfate is a decapeptide with antibacterial activity mainly against gram-negative micro-organisms (27). In the present study, the combined use of SB and reduced antibiotics was introduced given the inconsistent effects of SB fed alone and the still widespread use of antibiotics in many countries. Alterations with regard to intestinal barrier function may explain the improved performance and decreased diarrhea incidence by the ASB and PC treatments.
We first validated that the ASB treatment improved performance in weaned piglets with effects comparable to those for the PC treatment. This observation is similar to that in a previous study (17), which reported that a combination of acidifiers and avilamycin gave better performance-promoting effects than did acidifiers or avilamycin fed alone in piglet diets, although the mechanisms were not well determined. The decreased diarrhea incidences in the ASB and PC piglets were at least partly due to the redefinition of the bacterial communities by the antibiotics. In addition, SB was speculated to influence the intestinal microflora (14) and bacteria virulence (5) in weaned piglets. Our experiment revealed an effective strategy to control postweaning problems with reduced use of antibiotics.
The decreased digestion and absorption of nutrients, fluids, and electrolytes due to villous atrophy and crypt hypertrophy as a result of early weaning may contribute to diarrhea (28). In the present study, the improved villus height in the duodenum and ileum in the PC piglets may partly explain the improved performance and lower diarrhea incidence by the PC treatment. An increase in crypt depth can be used as a predictor of increased crypt cell production rate and overall stimulation of cell turnover in the small intestine, which are generally associated with reduced digestive and absorptive capacity (29). The decreased crypt depth in the duodenum, jejunum, and ileum in the ASB piglets was in agreement with the improved performance.
Increased intestinal secretion or losses of fluid and electrolytes may be considered as a final common pathway of diarrhea production, and many factors interact in subtle ways in the development of diarrhea (28). Increased intestinal permeability is characterized by downregulation of fluid and electrolyte absorption, which leads to fluid and electrolyte accumulation in the bowel (24). In our study, the decreased small intestinal permeability by the ASB treatment may help to confer a decrease in diarrhea incidence. The intercellular tight junction protein complexes play an important role in the mucosal barrier against translocation of intraluminal toxins, antigens, and enteric flora from the lumen into subepithelial tissues and systemic blood circulation (30). Note that the higher expression of occludin in jejunal tissue by the ASB treatment was consistent with the decreased small intestinal permeability observed. The ASB and PC treatments might also decrease paracellular permeability in the hindgut as indicated by the increased expression of occludin in colonic tissue. Previous studies showed that absorption in the large intestine is also involved in the pathophysiology of diarrhea, and a loss of fluid and electrolytes in the small intestine will only cause diarrhea when remedial reabsorption by the large intestine partly or completely fails (31). Collectively, the ASB and PC treatments decreased diarrhea incidence by modulating permeability in both the small intestine and hindgut.
Mucosal oxidative stress has been shown to be sufficient to induce diarrhea and to play an important role during the progression of weaning diarrhea (32). It is likely that the ASB triggered a localized form of attenuation in antioxidant status that was not reflected in the extraintestinal environment due to minor changes in serum antioxidant variables. This discrepancy could be explained by the negligible finding of butyrate in the peripheral blood as a result of rapid metabolism in the gut wall and/or in the liver (5). Glutathione peroxidase is implicated in the protection of gastrointestinal mucosal cells against damage from various insults (32). Malondialdehyde concentrations in tissues and blood are generally used as biomarkers of endogenous lipid peroxidation and free radical-induced damage (33). The increase in glutathione peroxidase in the intestinal mucosa could mainly be attributed to SB, as suggested by the absence of an increase in intestinal mucosal glutathione peroxidase content in the PC piglets, whereas antibiotics are mainly responsible for malondialdehyde decreases in intestinal mucosa. Decreased oxidative stress may lead to reduced damage to the intestinal mucosal barrier, which subsequently leads to reduced intestinal permeability.
Some cytokines are thought to be critical in the predisposition to and exacerbation of some gastrointestinal dysfunctions (34). TNF-α is a central mediator of intestinal inflammatory diseases (34) and has been shown to play a role in the control of intestinal permeability (35). T cell–derived TNF-α inhibits phosphorylation of the myosin light-chain mediated by the myosin light-chain kinase, leading to alternatively disrupted tight junction stability and dysregulation of occludin expression (36). Therefore, the increased occludin expression in the ASB and PC piglets could be partly attributed to decreased TNF-α in the intestinal mucosa. Moreover, TNF-α mediates mast cell degranulation along with some other mediators. TNF-α is, in turn, rapidly released by mast cells after degranulation as are some other inflammatory mediators (24). Mast cell degranulation and the consequent histamine and prostaglandin release profoundly influence intestinal epithelial barrier function (28). The 6-fold decrease in intestinal mucosal TNF-α may indicate an amelioration of mast cell degranulation in the ASB and PC piglets. More research is needed to explain the unexpected increase in serum IFN-γ, whereas the IFN-γ concentration in the intestinal mucosa was not affected by the ASB and PC treatments.
An understanding of how the ASB and PC treatments affect the intestinal bacterial communities may help to reveal the linkages between their changes and improvements in performance and diarrhea incidence. Higher bacterial diversity may be associated with an improved competitive defense against pathogens and decreased vulnerability to environmental perturbations, such as shifts in gut microbial communities, changes in piglet diet, or overt pathogenic challenge (37). Shiga toxin–producing Escherichia coli elicits fluid and electrolyte accumulation in the bowel by disrupting the usual balance of intestinal absorption and secretion toward net secretion (38). The increased bacterial diversity in the colonic lumen and decreased Enterobacteriaceae (mainly Shigella) in both the ileal and colonic lumen may help to decrease diarrhea incidence in ASB and PC piglets.
The colonic lumen harbored a higher number of bacteria associated with degrading complex carbohydrates than did the ileal lumen, which was consistent with previous observations (23, 39). The intestinal bacterial communities may participate in modulation of piglet performance due to the energy derived from hindgut bacterial fermentation. Bacteroidetes is well known for its role in polysaccharide degradation (40). Ruminococcaceae has been consistently found in the hindgut of pigs, and an increase in Ruminococcaceae may improve feed conversion in piglets due to its cellulose-degrading capacity (39). Lachnospiraceae is known to produce an array of bacteriocins and butyrate, and many isolates have shown promise as probiotics (41). In our study, the increases in the relative abundance of Bacteroidetes, Ruminococcaceae, and Lachnospiraceae in the colonic lumen of ASB and PC piglets could help the host obtain more energy from complex polysaccharides that are resistant to the action of digestive enzymes. Whereas the decrease in Lactobacillaceae populations in the ileal lumen may reduce energy losses, previous studies showed that Lactobacilli is the main contributor to microbial bile salt hydrolase activity in the small intestine, which results in impaired lipid absorption by the host animal and consequent dietary energy losses (42). In addition, we propose a hypothesis that the ASB and PC treatments improved the ratio of weight gain to feed via inhibition of the normal microbiota, leading to increased nutrient utilization and a reduction in the maintenance costs of the gastrointestinal system (43). By contrast, an undesirable effect may be the substantially increased Clostridiaceae population in ASB and PC piglets. The persistence of Clostridiaceae in the whole intestinal bacterial communities in ASB and PC piglets and their effect on piglet growth cannot be explained because the possible benefits from intestinal colonization by nonpathogenic Clostridiaceae have not been explored (44).
In summary, the present study showed that the ASB significantly improved weight gain and reduced diarrhea incidence in weaned piglets (P < 0.05). These changes were accompanied by decreased small intestinal permeability, increased intestinal occludin expression, and improvements in some antioxidant variables and immune indexes, as well as considerable alterations in intestinal bacterial communities.
Acknowledgments
We thank Katrina Mohror, South Dakota State University, for excellent assistance in editing this manuscript. C Huang, PS, and XM designed and conducted the research; C Huang, PF, C Hou, and XM analyzed the data; C Huang and XM wrote the manuscript; PT critically reviewed the manuscript; and XM had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
-
↵1 Supported in part by the National Basic Research Program of China (973 Program, 2013CB117301), the National Natural Science Foundation of China (31528018, 31272448, and 31472101), the National Department Public Benefit Research Foundation (201403047), and Beijing Nova program (xx2013055).
-
↵2 Author disclosures: C Huang, P Song, P Fan, C Hou, P Thacker, and X Ma, no conflicts of interest.
-
↵3 Supplemental Table 1 is available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
-
↵7 These authors contributed equally to this work.
-
↵8 Abbreviations used: ASB, reduced antibiotics + sodium butyrate; NC, negative control; PC, positive control; SB, sodium butyrate.
- Manuscript received: June 3, 2015.
- Initial review completed: June 23, 2015.
- Revision accepted: September 29, 2015.
Sodium butyrate in piglet feed reduces diarrhoea
Sodium butyrate in piglet feed reduces diarrhoea
In a recent paper (J. Anim. Sci. 2012), Ma et al. tried to understand by which mechanism sodium butyrate can decrease the incidence of diarrhoea. In an in-vitro experiment, cultured cells were scratched to induce wound, then were treated with sodium butyrate. The publication shows that addition of sodium butyrate accelerated the process of wound healing, confirming a protecting effect of butyrate on the intestinal mucosa. When compared to a control group, the authors observed that mRNA expression of the intestinal mucosal tight junction proteins occludin and zonula occluden protein-1 was increased.
Besides, in the butyrate-treated cells, the levels of superoxide dismutase and glutathione peroxidase, two of the main antioxidant enzymes, as well as glutathione, one of the nonenzymatic antioxidant components, were significantly higher than in the control group. Also, malondialdehyde, a marker of free radical mediated lipid peroxidation injury, was present at lower levels in the cells treated with sodium butyrate. These results confirm previous research suggesting that one of the benefits of sodium butyrate in the intestine is to maintain the function of the intestinal barrier.
Together with the report of Peng et al. (2009), this study also indicates that adding sodium butyrate in feed can probably play an important role in maintaining the intestinal tight junctions, which are essential to preserve the gut integrity.