https://www.cambridge.org/core/services/aop-cambridge-core/content/view/S0954422410000247
http://www.feedadditive.com/docs/S0954422410000247.pdf
Absorption of butyrate, SCFA membrane receptor and transport proteins
Earlier studies have demonstrated that SCFA are absorbed in both the small and large intestine by similar mechanisms(65,66). More recent studies suggest the existence of species differences and different transporter isoforms expressed in enterocytes along the intestine(67). On the apical membrane of the epithelial cell, distinct transport mechanisms have been reported, i.e. non-ionic diffusion and mechanisms involving SCFA/HCO3 2 exchangers, monocarboxylate transporter (MCT) type 1(68,69), and Na-coupled MCT(70) (SMCT or SLC5A8/12). On the basolateral membrane, a carrier-mediated, HCO3 2-gradient-dependent anion–butyrate exchange system (Fig. 2) is found(71). The human intestine constitutes MCT3, MCT4 and MCT5 isoforms (the MCT6 expression was not found), with low expression of MCT3 in the ileum, and high expression of MCT4 and MCT5 found predominantly in distal colon(67). In ruminant species, the SCFA-transporting mechanisms in the rumen epithelium are highly efficient, and lead to absorption of nearly all volatile fatty acids. Anion competition experiments in the washed ovine reticulorumen segments revealed that SCFA can be transported by both bicarbonate-dependent and bicarbonate-independent protein-coupled mechanisms(72). The latter was not coupled with MCT. MCT are involved in butyrate transport in pig and human colonic luminal membrane. Ritzhaupt et al. (68,69) demonstrated that butyrate uptake is via a pH-activated, electroneutral anion exchange system. The optimal pH for the activity of the colonic butyrate transporter seems to be 5·5. Butyrate transport with MCT is saturable, coupled with Hþ and inhibited by several monocarboxylates such as acetate, propionate, pyruvate, L-lactate and a-ketobutyrate. More recently, a second class of MCT was identified(70), named SMCT. Two proteins have been cloned: SLC5A8 or SMCT1 and SLC5A12 or SMCT2(73). Conversely to MCT, SMCT transport mechanism involves Naþ uptake by the transport cycle and also uses nicotinate and ketone bodies as substrates. In the colon, Li et al. (17) showed that SLC5A12 (or SMCT2) functions as a tumour suppressor. Ganapathy et al.(70) demonstrated that a non-malignant colon cell line expresses the transporter contrary to malignant cells. So, exposure of non-malignant cells to butyrate does not induce apoptosis. However, when SLC5A12 (SMCT2) is ectopically expressed in malignant cells and when butyrate is added in the culture medium, cells undergo massive apoptosis. In the normal colon, SLC5A8 is claimed to have less importance in butyrate transport than MCT1(74) but it plays a key function in intestinal lactate absorption. In humans, SLC5A8 is considered a tumour suppressor(75). During the transformation of non-malignant cells into malignant ones, expression of SLC5A8 is silenced perhaps to avoid butyrate inhibition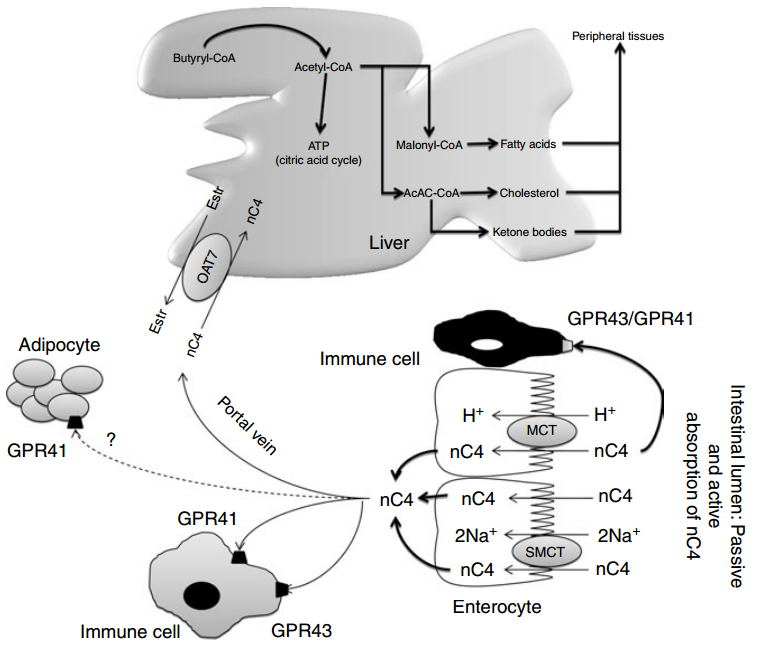
Fig. 2. Absorption of n-butyrate (nC4) in the large intestine and subsequent metabolism. Butyrate transport with monocarboxylate transporters (MCT) is saturable and coupled with Hþ transport. Several receptors for butyrate have been identified and detected in a variety of tissues including fat tissue, but the highest expression has been found in immune cells. Butyrate prevents obesity and decreases body fat mass in mice, but the exact mechanism is unknown. After the intestine, butyrate can be metabolised in the liver to produce fatty acids, cholesterol and ketone bodies. In the peripheral blood, no significant concentration of butyrate is found. Oestr, oestrone sulfate; OAT7, organic anion transporter 7; GPR, orphan G protein-coupled receptor for SCFA; SMCT, Na-coupled monocarboxylate transporter. Adapted from Gupta et al.
of histone deacetylase. But in the presence of butyrate, expression of SLC5A8 is again enhanced and it increases cell apoptosis(75). Nevertheless, the lack of susceptibility for colon cancer in SLC5A8 2 /2 mice and the failure to detect a significant uptake of butyrate by SLC5A8 question the role of SLC5A8 both in butyrate transport and colon cancer suppression(76). Two orphan G protein-coupled receptors (GPR) for SCFA, GPR41 (or FFA3) and GPR43 (or FFA2) have been isolated from the intestine(77 – 82). FFA2 mRNA was detected in a variety of tissues, but the highest expression was found in immune cells, including polymorphonuclear cells, suggesting that SCFA might be involved in the activation of leucocytes. Indeed, recent studies in (FFA2) GPR43- deficient mice (Gpr432/2) indicated that acetate administration may help in resolution of the inflammatory response(83). FFA3 has an even more widespread expression pattern than FFA2, including adipose tissues, pancreas, spleen, lymph nodes, bone marrow and peripheral blood mononuclear cells(81). Abundant FFA2 and FFA3 expression was detected in the rat distal ileum and colon, and the human ascending colon(78,84,85). Expression was limited to mucosal cells, and absent in enteric neurons and smooth muscle cells. Immunohistochemical staining with antiFFA2 and anti-FFA3 sera showed FFA2 and FFA3 immunoreactivity in enterocytes and enteroendocrine cells of open type(78,86). The FFA3 immunoreactive enteroendocrine cells were less numerous than the FFA2 cells, and double-immunostaining for FFA2 and FFA3 revealed no common localisation in one cell. Both the FFA2- and FFA3- immunoreactive enteroendocrine cells exhibited peptide YY, but no 5-hydroxytryptamine, expression, supporting the evidence for stimulation of peptide YY release by SCFA(78). The FFA2 and FFA3 distribution and physiological role in the GIT, involving sensing of luminal content, intestinal motility, secretion and innate immunity, have been recently reviewed by Karaki & Kuwahara(86). In healthy rats fed a fibre-free or a RS-enriched diet and using [1-13C]butyrate intra-caecal perfusion, the flux of butyrate production was 0·7–1·8mmol/min whereas portal flux was from 0·2 to 0·4 mmol/min and parietal utilisation from 0·9 to 1·8mmol/min(87). In unfed pigs with an intracaecal perfusion of [1-13]C-butyrate, the parietal utilisation of butyrate varied from 60 to 120 mmol/min when the concentration of perfused [1-13]C-butyrate varied from 0 to 160 mmol/min (L Martin, unpublished results). Energy source for colonocytes In vitro studies have demonstrated that butyrate also represents the preferred energy-providing substrate for the colonic cells(4,88). Colonocytes exhibit a great capacity to rapidly metabolise butyrate. Through fatty acid oxidation, butyrate is entirely oxidised into CO2 or used as a precursor for lipid synthesis(89). In fact, butyrate is able to increase lipogenesis from acetyl-CoA or ketone bodies synthesis via the hydroxyl-methyl-glutaryl-CoA pathway(90). Consequently, the synthesis of many key components of the intestinal epithelial tissue depends on butyrate metabolism (see later). As butyrate is a key substrate for colonocytes, very small quantities reach the general circulation or portal vein. Guilloteau et al. (8) did not observe any changes in plasma butyrate concentration in the peripheral circulation in calves. Nevertheless, the concentration of butyrate in the portal vein may vary according to the diet. Some substrates such as RS or oligosaccharides lead to substantial increases in butyrate concentration in the portal vein(91,92). Hepatic uptake of butyrate is almost total. In the liver, butyrate metabolism also yields acetyl-CoA as in colonocytes. In contrast to single-stomached animals, in vivo studies in steers revealed important butyrate uptake in the rumen as well as limited capacity to metabolise butyrate in the ruminal epithelium and liver. In cattle, butyrate absorption is saturable and if it exceeds the metabolic capacity, it affects rumen epithelial, hepatic nutrient metabolism and the nutrient supply of peripheral tissues(93).
Butyrate transport into the peripheral blood circulation: is there proof for physiological direct effects?
As shown by Kristensen & Harmon(93) in cattle, substantial amounts of butyrate produced by rumen microbiota may be absorbed through the ruminal epithelium and induce a number of effects via the blood circulation. First, included at a low dose in the diet (0·3 % of DM), butyrate disappears from the upper GIT (mainly in the stomach). It is thought to be metabolised in the GIT wall because it is not found in blood(8,94). Nevertheless, when ingested with the diet, in specific conditions, butyrate would be measurable in peripheral blood(95) and seems to be able to act on peripheral organs (skeletal muscle, brown adipose tissue, liver, etc). Second, butyrate generated from dietary fibre fermentation at a high dose in the hindgut lumen (from 3 to 70 mM) (96) is quickly absorbed in rodents(97) and transported via the portal vein to the liver. In humans the butyrate concentration in the portal vein is about 30 mM whereas it is 12 mM in the hepatic vein(1). A significant amount of butyrate is detected in the portal vein but not in the peripheral circulation in pigs fed a diet enriched with resistant potato starch(98). Similarly, using rye fibres as a RS source, butyrate concentration significantly increases in both the portal vein and peripheral circulation(99). In pigs, the direct perfusion of sodium butyrate in the caecum induces a dose-related increase of butyrate in the portal blood (L Martin, unpublished results). Butyrate, however, is not regularly detected in the peripheral blood even after a long period of perfusion with a supra-physiological solution of butyrate in the colonic lumen (L Martin, unpublished results). Taken together, these data suggest that butyrate can be absorbed from the gut and entirely metabolised either in the gut mucosa or in the liver, which makes a direct effect of butyrate via blood circulation unlikely. Indeed, Bloemen et al. (100) showed that in human patients, no intestinally produced butyrate escaped the splanchnic area due to a highly efficient hepatic uptake.
Butyrate and hepatic metabolism
Only a small fraction of luminal butyrate can reach the liver via the portal vein. Nevertheless, the effect of butyrate on hepatic cell has been studied. In the liver, butyrate leads to The multiple effects of butyrate 371 Nutrition Research Reviews https:/www.cambridge.org/core/terms. https://doi.org/10.1017/S0954422410000247 Downloaded from https:/www.cambridge.org/core. IP address: 113.52.96.90, on 30 Apr 2017 at 03:20:19, subject to the Cambridge Core terms of use, available at the production of acetyl-CoA that enters into the citric acid cycle. It has been shown that the production of acetyl-CoA via the butyryl-CoA synthase pathway consumes 1 ATP/mol but due to the reoxidation of reduced compounds, the tissue ATP content is maintained. In an experiment using isolated perfused liver of rats, Beauvieux et al. (101) observed that, unexpectedly, butyrate perfusion led to an impairment in energy metabolism, i.e. a decrease in net ATP content in comparison with acetate. The authors hypothesised that butyrate might impair mitochondrial activity inducing an uncoupling between the respiration chain and ATP synthesis. So, the effect of butyrate on hepatic metabolism appears to be close to that of longer-chain fatty acids. As long-chain fatty acids play a part in the pathogenesis of insulin resistance, the effect of butyrate should be considered in specific nutritional conditions (acarbose treatment, high level of dietary RS, etc)(102). When ingested, butyrate enhances glycogen synthesis in the liver at the same rate as glucose(103). The authors demonstrated a clear effect on both a decrease in glucose oxidation and an increase in hepatic glycogen storage. They postulated that such a mechanism could be one of the molecular bases to explain the effect of dietary fibres on the prevention of insulin resistance. Recently a new organic anion transporter (OAT7) was specifically identified in the liver(104), exhibiting a significant transport activity for butyrate (Fig. 2). This transporter mediates the bidirectional transport of oestrone sulfate in exchange for butyrate. This exchange with oestrone sulfate might suggest a contribution (direct or indirect) of butyrate in liver steroid hormone metabolism. More fundamentally, the narrow substrate selectivity of OAT7 suggests that butyrate might participate in the efficient translocation of some sulfate conjugates without interference from the other anionic compounds such as bile salts. In other words, butyrate may play a role in the efficiency of liver detoxification pathways.